Clinical Studies
Our clinical studies research determines the activities of key human pathogens in the infected human patient. Such data have never successfully been systematically investigated by drug development programs using traditional laboratory conditions, but they are crucial for targeting pathogens with novel control strategies under physiologically relevant conditions.
We achieve this goal by obtaining patient samples that are freshly frozen directly at the bedside, and analyse these samples using ultrasensitive mass spectrometry and other cutting-edge analytical methods. This includes quantifying pathogen protein as well as metabolites, antimicrobials, and host macromolecules that shape the in vivo microenvironments.
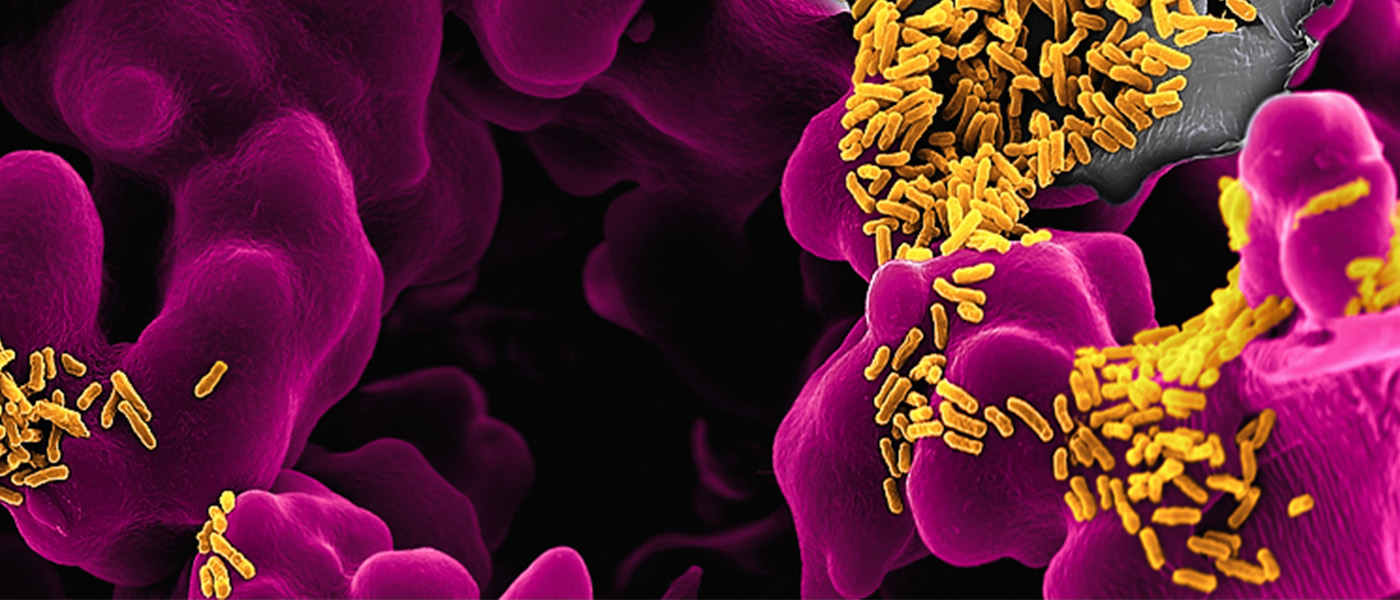
The results provide informative benchmarks for evaluating and optimising patient-mimicking in vitro models. In addition, the data will reveal commonalities and variation in properties of diverse pathogen strains in human host tissues in the presence of various comorbidities. Initial data demonstrate that both goals are attainable with recently established clinical studies and analytical workflows.
Our three focus approach
- To extend ongoing clinical studies for obtaining freshly frozen patient samples
- To determine human components and pathogen properties in these samples
- To derive guidance and benchmarks for development of patient-like in vitro models
Leadership of Clinical Studies research

Leader:
Nina Khanna »

Co-leader:
Dirk Bumann »